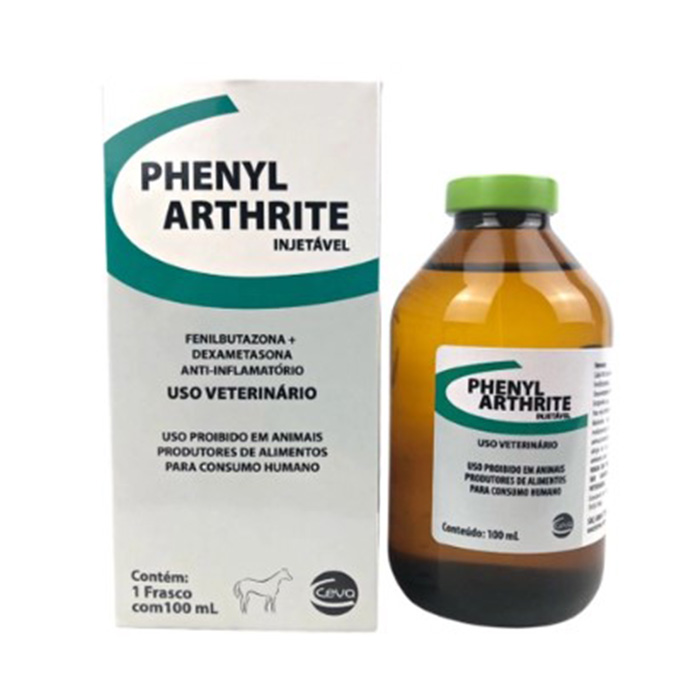
Phenylarthrite – 100 ml (Ceva Lab)
€72,60
Additional information
COMPOSITION
Each 100g of Injectable Phenylarthrite contains:
Phenylbutazone – 18 g
Dexamethasone (Sodium Phosphate) – 18 g
Excipients q.s. – 100 ml
| Weight | 275 g |
|---|---|
| Dimensions | 15 × 10 × 10 cm |
Description
Product Description
Phenylarthrite shows a true potentiation of the anti-inflammatory effects of the drugs alone.
Thanks to phenylbutazone, there is a strong inhibition of the action of the enzyme cyclooxygenase (COX-2) in the production of mediators of inflammation. Dexamethasone, on the other hand, inhibits the expression of COX-2, inhibiting the transcription of the relevant gene and, in this way, reduces the generation of prostaglandins responsible for the symptoms of inflammation.
CONTRAINDICATIONS:
The use of corticosteroid preparations in acute infections should be avoided. When used in non-acute infectious conditions, treatment should be associated with bactericidal antibiotics to the detriment of bacteriostatic antibiotics.
In general, corticosteroids are contraindicated in viral infections and late in pregnancy, when they can induce labor. They should also not be administered to animals that have renal, cardiac, and hepatic insufficiency, or gastric ulcer, and that are hypersensitive to the components of the formula. Its use is not recommended in animals with diabetes mellitus, osteoporosis, and hemato cytological alterations (aplastic anemia, leukopenia, agranulocytosis, and thrombocytopenia). Do not apply to newborn animals.
Treatment duration:
Use higher doses for the first 48 hours, then the dose is gradually reduced to a maintenance level and is maintained at a lower level capable of achieving a desired clinical response. Treatment varies from 3 to 14 days (an average of 7 days in horses) and 3 days in canines.
Drug interactions:
There are risks of drug interactions occurring when Phenylbutazone is administered with other non-steroidal anti-inflammatory agents, as they have a similar mechanism of action (cyclooxygenase inhibition) which may increase the occurrence of adverse effects.
Withdrawal period for the Injectable Phenylarthrite product:
Cattle: Slaughter: 14 days. Milk: 05 days.
Pigs: Slaughter: 14 days.
Horses: This product must not be applied to horses intended for slaughter or human consumption.
Phenylarthrite is indicated for horses, camels, cattle, pigs, and dogs, where it is recommended to combat painful and feverish states and inflammatory manifestations, such as arthritis, bursitis, myositis, joint and muscular rheumatism, congestive processes, tendinitis, and trauma.
DOSAGE AND ADMINISTRATION
Intravenous injections are preferred over intramuscular administration, as phenylbutazone may cause irritation at the application site. Inject slowly intravenously at a rate of 10ml every 30 seconds.
Adult horses: 3 to 4 ml of Injectable Phenylarthrite for every 100 kg of body mass, by slow intravenous route, every 24 or 48 hours.
Adult Camels: 2 to 3 ml of Injectable Phenylarthrite for every 100 kg of body mass, by slow intravenous route, every 24 or 48 hours.
Foals: 1 to 2 ml of Injectable Phenylarthrite for every 50 kg of body mass, by slow intravenous route, every 24 or 48 hours.
Dogs: 1ml of Injectable Phenylarthrite for every 15 kg of body mass, by the slow intravenous route, daily. The maximum daily dose should not exceed 800.00 mg.
Cattle: 1 to 2ml of Injectable Phenylarthrite for every 100 kg of body mass, by deep intramuscular route, every 48 hours.
Pigs: 0.5 to 1.5 ml of Injectable Phenylarthrite for every 50 kg of body mass, intramuscularly (single dose).
PRESENTATION:
100 ml




Reviews
There are no reviews yet.