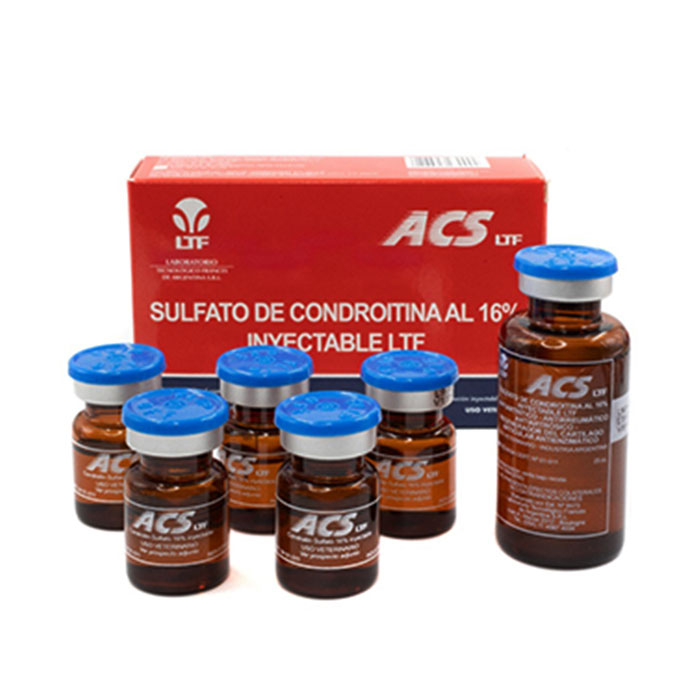
Acs Ltf 16% -25ml
€74,80
Additional information
| Weight | 90 g |
|---|---|
| Dimensions | 11 × 6 × 6 cm |
Description
Product Description
ACS LTF 16% INJECTABLE CHONDROITIN SULFATE
Antiarthritic – Antirheumatic – Antiarthritic
Regenerator of articular cartilage – Antiezimático
Regeneration of Cartilage in Shock Treatment.
Even the most incipient cartilage problems cause pain that threatens the performance of horses exposed to high demands.
ACS LTF – 16% Chondroitin Sulfate Acid – is a habitual and highly recognized product in San Isidro, Palermo, and La Plata racecourses where Carrera Pure Bloods find a highly effective relief reaching its maximum performance. The pain happens because ACS LTF remits the injury. Other sport horses, such as Trotadores, Salto, Polo, and Endurance, also maintain optimal legs and hands thanks to ACS LTF 16%
Its extension to the treatment of cartilage problems in dogs and cats has been highly efficient, especially with sheep and large animals.
The injectable formula allows the initiation of treatments with rapid effects, which can be continued in the same way or with oral ACS LTF. (See technical notes)
Formula
Chondroitin sulfate
Sodium salt dihydrate: 16 g.
Thimerosal: 0.003 g.
Sterile injectable water C.S.P .: 100 ml.
Therapeutic Action
Arthritis, osteoarthritis, coxarthrosis, hip dysplasia, osteoarthrosis, chondropathies, post-operative fractures, osteochondrosis, chondroprotective and chondroreparative. Hydrarthrosis, capsulitis, synovitis, osteitis peri plantar, and angular of 3rd phalanx.
Spondylosis, ankylosing spondyloarthritis, calcification of the intervertebral discs, loose carpus syndrome in large breeds, ptosis in pseudoescorbuto ears, chondrodystrophic in the postoperative period of aesthetic otoplasty, genu valgum, feline fibrous osteodystrophy, tendon repairs, joint postoperative, desmitis, tendonitis, Degenerative joint disease (EDA).
Dosage and method of administration
Given the safety of the product, it is recommended to administer an application at intervals of 1 to 4 days according to the severity of the present lesion and the criteria of the acting physician.
Horses: Intramuscular route
Each 100 Kg PV ……………………. 1 cc
10 average total applications are suggested.
Canines and Felines: Subcutaneous and/or intramuscular route.
Up to 10 Kg of PV ………….. 1 cc
From 10 to 30 Kg of PV ……… 2 cc
Of more than 30 Kg of PV ….. 2.5 cc
Given the excellent safety margin of the active principle and its excipients, the doses and time of treatment will be according to the criteria of each acting Veterinarian.
Details
Collateral effects: does not present.
Conservation: between 5º and 35º C. Sheltered from direct sunlight.
Contraindications
It does not present.
Side effects: does not present.
Presentation
Bottle x 25 ml.




Reviews
There are no reviews yet.